Question
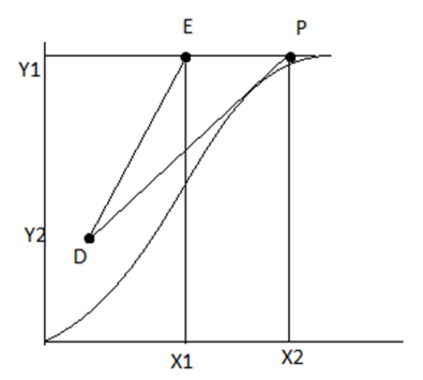
For the operating line DE minimum liquid is required.
a.
True
b.
False
c.
May be True or False
d.
Can't say
Posted under Mass Transfer
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
Q. Have a look on the below diagram. Here, DE and DP are the operating line. Also Y and X are the concentrations in mole ratio for gas and liquid phase. It’s clearly a representation...
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. Find the slope of the operating line.
Here, the concentrations are represented in mole ratio. The value of (Y1-Y2)/(X1-X2) =2. Find the slope of the operating line.
View solution
Q. Find the representation,
Where, Y- gas phase concentration in mole ratio
X-liquid phase concentration in mole ratio
View solution
Q. Kremser brown equation will be invalid for gas phase transfer units if operating and equilibrium line has same slope.
View solution
Q. Find the HTU if gas rate is 0.07 kmol/sq.m s and the Fg* a ( F type mass transfer co-efficient) is 0.06 kmol/ cu.m s.
View solution
Q. Find the absorption tower height if
Number of gas phase transfer units 4.09
Height of gas phase transfer units 1.5 meters
View solution
Q. Will the Height equivalent theoretical plates changes with flow rates?
View solution
Q. Packing height in the packed tower = NTU x __________
View solution
Q. Packed towers are _____________
View solution
Q. Absorption factor increases equipment cost decreases
View solution
Q. The equation helps us to find the number of trays in an absorber theoretically is _________
View solution
Q. Find the improper characteristic of ideal solution.
View solution
Q. Find the most common example for absorption.
View solution
Q. Find the false statement for the better choice of the absorbent.
View solution
Q. Match the following:
1) Hendry’s law a) Ideal solution
2) Dalton’s law b) Non- Ideal solution
3) Raoult’s law c) Sum of partial pressure
View solution
Q. According to Hendry’s law,
View solution
Q. According to Raoult’s law, for a pure component solution the partial pressure is equals to
View solution
Q. Which of the following is not an example of ideal solution?
View solution
Q. Solubility of a gas increases with increase in temperature.
View solution
Q. The equilibrium characteristics of the solubility of a gas in liquid helps to determine the
View solution
Q. Gas absorption is the process of transferring solute component from liquid solvent to gas mixture.
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Mass Transfer? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!








