Question
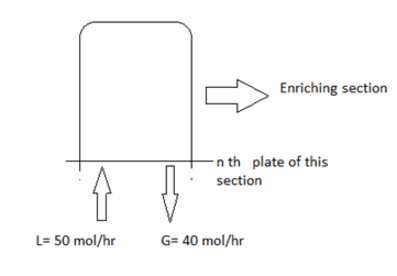
a.
0.8
b.
1.25
c.
1.2
d.
1
Posted under Mass Transfer
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
Q. Find the internal reflux ratio by analysing the below fractionator.
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. From the enthalpy-concentration diagram find the location of heat removed by the condenser.
View solution
Q. Find the location of condenser from the figure below.
View solution
Q. Look the below figure and find the possibility of measurement.
View solution
Q. Find the point P in this enriching section.
Where,
Xd- concentration of distillate and R- reflux ratio
View solution
Q. Express the reflux ratio.
View solution
Q. The notation X from the figure below is known as Reflux.
View solution
Q. Find the distillate rate, if a differential condenser is used and the equilibrium relation is given as x*= 0.2y. Also the rate of feed is 100 mol/hr.
View solution
Q. Find the value of relative volatility for a constant pressure process if
View solution
Q. Find the composited distillate composition.
View solution
Q. Find the feed rate by analysing the graph.
View solution
Q. Find the feed composition of more volatile component by analyzing the table below.
Hint: can neglect pre-heater.
View solution
Q. Find the X and Y from the flash column given below.
View solution
Q. Express the point A.
View solution
Q. Find the relative volatility, if x, y are the concentrations in mole fraction.
View solution
Q. Look out the distribution curve, if the line PM is lesser than shown the separation is more.
Where, x,y are the concentrations in mole fractions.
View solution
Q. By adding entrainer or solvent one of the component’s _________ gets reduced.
View solution
Q. Will it possible to separate the mixture having relative volatility value 2 with azeotropic distillation.
View solution
Q. If the feed mixture has the same vapour and liquid compositions at equilibrium then the distillation is normal.
View solution
Q. Find the Azeotropic mixture.
View solution
Q. In the distillate use of heat is to separate the distillate from the entrainer.
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Mass Transfer? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!








