Question
a.
1st order kinetics
b.
2nd order kinetics
c.
Mixed order kinetics
d.
Zero-order rate
Posted under Drug and Pharmaceutical Biotechnology
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
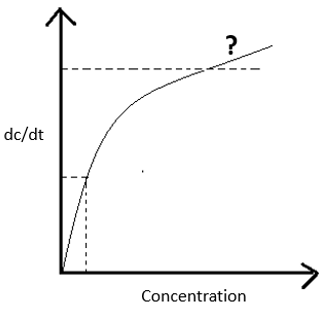
Q. In the given picture, which kinetic order the graph is following at the marked place?
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. In the given picture, which kinetic order the graph is following at the marked place?
View solution
Q. In the given picture, which kinetic order the graph is following at the marked place?
View solution
Q. Which equation plot is being shown in the picture?
View solution
Q. In the given picture, the marking “b” represents the drug concentration of which compartment?
View solution
Q. In the given picture, the marking “a” represents the drug concentration of which compartment?
View solution
Q. At which of the four marked points of the plasma drug concentration versus time graph, absorption rate = elimination rate?
View solution
Q. Which of the following is used in molecular encapsulation of drugs for enhancing bioavailability?
View solution
Q. Which of the following is used in solid solutions method of enhancing bioavailability?
View solution
Q. Which of the following is used in the solvent deposition method of enhancing bioavailability?
View solution
Q. Which one of the following is used for selective adsorption on insoluble carriers of the drugs?
View solution
Q. How pH alteration of the drug microenvironment is done?
View solution
Q. Salts improve solubility and dissolution characteristics.
View solution
Q. What is the principle behind the use of surfactants?
View solution
Q. What will be the particle size after micronization of drugs?
View solution
Q. Which of the following is not an approach for overcoming bioavailability problems?
View solution
Q. A drug with poor stability means higher bioavailability.
View solution
Q. Poor bioavailability means poor aqueous solubility.
View solution
Q. Bioequivalence is a relative term which denotes that the drug substance reaches the systemic circulation at the same relative rate or time.
View solution
Q. If the bioavailability of test formulation is 80-120% of the reference standard, it is considered to be bioequivalent.
View solution
Q. Two or more drugs contain the same therapeutically active ingredient which elicits same pharmacological effects and can control the disease to the same extent are known to be pharmaceutic equivalent.
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Drug and Pharmaceutical Biotechnology? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!








