Question
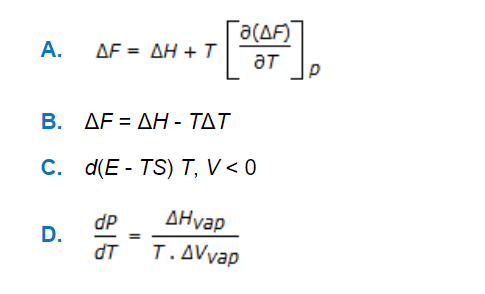
a.
A
b.
B
c.
C
d.
D
Posted under Basic Chemical Engineering
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
Q. Gibbs-Helmholtz equation is
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. Which of the following identities can be most easily used to verify steam table data for superheated steam.
View solution
Q. An ideal monoatomic gas is taken round the cycle ABCDA as shown below in the P-V diagram. The work done during the cycle is
View solution
Q. Which of the following is Virial equation of state?
View solution
Q. Chemical potential of ith component of a system is given by
View solution
Q. The following equation, applicable to a binary solution of components. A and B in equilibrium with their vapors at constant temperature and pressure is called the __________ equation.
View solution
Q. Which of the following represents the Virial equation of state ?
View solution
Q. The equilibrium constant for a chemical reaction at two different temperatures is given by
View solution
Q. Joule-Thomson co-efficient is defined as
View solution
Q. To obtain integrated form of Clausius-Clayperon equation given by following equation, from the exact Clayperon equafion, it is assumed that the
View solution
Q. Trouton's ratio is given by (where λb, = molal heat of vaporisation of a substance at its normal boiling point, kcal/kmol Tb = normal boiling point, °K )
View solution
Q. Entropy of an ideal gas depends upon its
View solution
Q. Isobaric process means a constant process.
View solution
Q. No work is done by the system, when a reaction occurs at constant
View solution
Q. If the vapour pressure at two temperatures of a solid phase in equilibrium with its liquid phase are known, then the latent heat of fusion can be calculated by the
View solution
Q. The freezing point of a liquid decreases when the pressure is increased, if the liquid __________ while freezing.
View solution
Q. In a reversible chemical reaction (where, Δx = number of moles of products-number of moles of reactants )
View solution
Q. Out of the following refrigeration cycles, which one has maximum COP ?
View solution
Q. Pick out the correct statement:
View solution
Q. In any spontaneous process,
View solution
Q. Which of the following is a thermodynamic property of a system ?
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Basic Chemical Engineering? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!








