Question
a.
I, II, III
b.
III, II, I
c.
III, I, II
d.
II, III, I
Posted under Basic Chemical Engineering
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
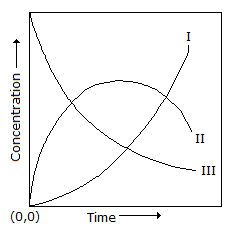
Q. A first order homogeneous reaction of the type X → Y → Z (consecutive reaction) is carried out in a CSTR. Which of the following curves respectively show the variation of the...
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. In a chemical reaction given below , it is observed that the
(i) rate of formation of 'P' is doubled on doubling the concentration of 'X'.
(ii) rate of formation of 'P' is quadrupled on doubling the concentration of 'Y'.
(iii) doubling the concentration of 'Z' does not affect the rate of formation of 'P'.
What is the order of the above chemical reaction?
View solution
Q. Transition state theory relates the above quantities as
View solution
Q. For a first order reaction carried out in a plug flow reactor, the space time is
View solution
Q. Second order consecutive irreversible reactions given below were carried out in a constant volume isothermal batch reactor with different initial feed compositions. Reactor temperature was same in all the cases. In experiments where the ratio of concentration of B to that of A in the initial feed was less than 0.5, the concentration of B increased first, reached a maximum and then declined with time. However, for all experiments where this concentration ratio was 0.5 or above, concentration of B decreased monotonically with time right from the beginning. What is the ratio of the two rate constants (k₁/k₂)?
View solution
Q. A consecutive reaction given below, is characterised by
View solution
Q. Pick the WRONG design guideline for a reactor in which the reactions, A → R (desired) and A → S (undesired) are to take place. The ratio of the reaction rates is as given below .
View solution
Q. In case of a consecutive unimolecular type first order reaction given below, the concentration of component __________ increases continuously with time.
View solution
Q. For the irreversible elementary first order reaction in parallel given below, a plot of Cy Vs. Cz will give a straight line having a slope of
View solution
Q. The equilibrium constant for the reversible reaction given below, is affected by the
View solution
Q. When the reaction is dominated by in-traparticle diffusion, the apparent order of reaction (nᴅ) as measured is related to the true order (n) as
View solution
Q. An elementary liquid phase decomposition reaction given below is to be carried out in a CSTR. The design equation is
View solution
Q. According to Arhenious equation of temperature dependency of rate constant for an elementary reaction
View solution
Q. The order of the following reaction is
View solution
Q. The half life period 't' of a zero order reaction given below, is equal to
View solution
Q. The point selectivity of the product 'Y' in the below reaction, is equal to
View solution
Q. The first order gas phase reaction given below, is conducted isothermally in batch mode. The rate of change of conversion with time is given by
View solution
Q. The rate of a following autocatalytic reaction, is given by -rᴀ = k . Cᴀ . Cʙ. In this case, the
View solution
Q. For the reaction given below, the rate of formation of Z is 0.2 gm mole/litre.hr. what is the rate of disappearance of X in gm mole/litre. hr ?
View solution
Q. Which of the following is not a dimension-less group used in catalysis ?
(where, D = dispersion co-efficient, cm² /sec.
D1 = diffusion co-efficient; cm²/sec.
L = length of the reactor, cm.
t = time, sec.
v = volumetric flow rate, cm³/sec .
V = volume, cm³.)
View solution
Q. Volume change for unimolecular type first order reaction given below, increases __________ with time.
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Basic Chemical Engineering? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!








