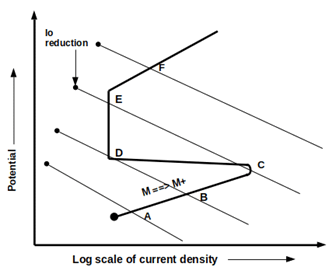
Question
a.
Effect of oxidizers concentration on cathodic behavior of active metal
b.
Effect of oxidizers concentration on the electrochemical behavior of active metal
c.
Effect of oxidizers concentration on the electrochemical behavior of active-passive transition metal
d.
Effect of oxidizers concentration on cathodic behavior of active-passive transition metal
Posted under Corrosion Engineering
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
Q. Which of the following is described in the given figure?
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. Which of the following statements is/are correct regarding the given schematic?
View solution
Q. What is depicted in the given polarization curve?
View solution
Q. Which of the following is depicted in the given mixed potential schematic?
View solution
Q. Which of the following type(s) of polarization is depicted in the given figure?
View solution
Q. Which of the following is/are correct regarding the given figure?
View solution
Q. What is depicted in the given figure?
View solution
Q. What is depicted in the given figure?
View solution
Q. Which of the following metal shows the highest exchange current density for hydrogen-hydrogen ion as per given figure?
View solution
Q. Which of the following zone(s) of given Pourbaix diagrams indicate a passive zone?
View solution
Q. Which of the following zone of given Pourbaix diagram indicates the immune zone?
View solution
Q. What is depicted in the given figure?
View solution
Q. Which of the following type of reaction is shown in the given figure?
View solution
Q. What is the Nernst equation for chemical reaction nA + mB ==> pC + qD at temperature T?
View solution
Q. What is the overall standard cell potential of a given Cu-Fe system?
View solution
Q. What are the cathodic and anodic reactions respectively of a given Cu-Zn system?
View solution
Q. Which of the following is/are correct regarding the given figure?
View solution
Q. Which of the following industries uses Tafel extrapolation or linear polarization to measure low corrosion rates?
View solution
Q. What is the formula to determine the slope of linear polarization curve?
View solution
Q. What is the range of current density around corrosion potential that will observe that the applied current density is a linear function of the electrode potential?
View solution
Q. Which of the following is/are the limitations of Tafel extrapolation?
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Corrosion Engineering? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!