Question
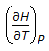
a.
CV
b.
entropy change
c.
Gibbs free energy
d.
none of these
Posted under Basic Chemical Engineering
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
Q. Following is the mathematical expression for
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. An ideal gas is taken around the cycle ABCA as shown in P-V diagram below. The work done by the gas during the cycle is equal to
View solution
Q. The Maxwell relation derived from the differential expression for the Helmholtz free energy (dA) is
View solution
Q. Gibbs free energy (G) is represented by, G = H - TS, whereas Helmholtz free energy, (A) is given by, A = E - TS. Which of the following is the Gibbs-Helmholtz equation
View solution
Q. Which of the following is clausius-Clayperon equation for vaporisation of an ideal gas under the condition that the molar volume of liquid is negligible compared to that of the vapor ?
View solution
Q. Which of the following is not a unit of the equilibrium constant Kp? (where, Δx = number of moles of products number of moles of reactants)
View solution
Q. Which is not constant for an ideal gas ?
View solution
Q. Following is the mathematical expression for
View solution
Q. The ratio of equilibrium constants (Kp₂/Kp₁) at two different temperatures is given by
View solution
Q. Consider the process A & B shown in the figure given below. In this case, it is possible that
View solution
Q. Joule-Thomson co-efficient which is defined by equation given below, changes sign at a temperature known as inversion temperature. The value of Joule-Thomson co-efficient at inversion temperature is
View solution
Q. The equation relating E, P, V and T which is true for all substances under all conditions is given by following equation .This equation is called the
View solution
Q. Cᵥ is given by
View solution
Q. The root mean square speed of molecules of a gas is equal to (where, m = mass of the molecule K = Boltzman's constant, T = absolute temperature)
View solution
Q. Efficiency of a Carnot engine working between temperatures T₁ and T₂ (T₁ < T₂) is
View solution
Q. A thermodynamic system is taken from state A to B along ACB and is brought back to A along BDA as shown below in the P-V diagram. The net work done during the complete cycle is given by the area covered by
View solution
Q. Maxwell's relation corresponding to the identity, dH = dS = Vdp + ∑μᵢ dnᵢ is
View solution
Q. Pick out the Claussius-Clayperon equation from the following:
View solution
Q. On a P-V diagram of an ideal gas, suppose a reversible adiabatic line intersects a reversible isothermal line at point A. Then at a point A, the slope of the reversible adiabatic line (∂P/∂V)s and the slope of the reversible isothermal line (∂P/∂V)T are related as (where, y = Cᵨ/Cᵥ)
View solution
Q. Gibbs-Helmholtz equation is
View solution
Q. Which of the following identities can be most easily used to verify steam table data for superheated steam.
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Basic Chemical Engineering? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!








