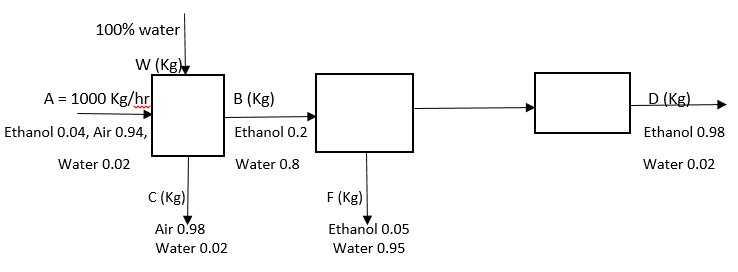
Question
What is the value of W?
a.
159.2 Kg/hr
b.
281.3 Kg/hr
c.
465.7 Kg/hr
d.
633.8 Kg/hr
Posted under Basic Chemical Engineering
Interact with the Community - Share Your Thoughts
Uncertain About the Answer? Seek Clarification Here.
Understand the Explanation? Include it Here.
Q. Answer the following question for the diagram given below. What is the value of W?
Similar Questions
Explore Relevant Multiple Choice Questions (MCQs)
Q. What is the value of C?
View solution
Q. Answer the following questions for the given diagram.
What is the value of B?
View solution
Q. What is the value of H/P in the following process, if 2P + H = 16?
View solution
Q. What is the value of F*P in the following process?
View solution
Q. What is the value of P in the following process?
View solution
Q. What is the value of P in the following process ?
View solution
Q. What is the value of P in the following process?
View solution
Q. Methane is at the rate 50 moles is supplied to a reactor with air at the rate 1000 moles, the reactor leaves O₂, N₂, CO₂ and H₂O, what is the percentage of H₂O in products?
View solution
Q. Methane is at the rate 50 moles is supplied to a reactor with air at the rate 1000 moles, the reactor leaves O₂, N₂, CO₂ and H₂O, what is the percentage of N₂ in products?
View solution
Q. Methane is at the rate 50 moles is supplied to a reactor with air at the rate 1000 moles, the reactor leaves O₂, N₂, CO₂ and H₂O, what is the percentage of CO₂ in products?
View solution
Q. Methane is at the rate 50 moles is supplied to a reactor with air at the rate 1000 moles, the reactor leaves O₂, N₂, CO₂ and H₂O, what is the percentage of O₂ in products?
View solution
Q. Methane is at the rate 50 moles is supplied to a reactor with air at the rate 1000 moles, the reactor leaves O₂, N₂, CO₂ and H₂O, what is the rate of products?
View solution
Q. octane is burnt with 40% excess O₂, what is the percentage of CO₂ in products?
View solution
Q. Propane is burnt with 20% excess O₂, what is the percentage of CO₂ in products?
View solution
Q. Pentane is burnt with 100% of excess air, what is the percentage of H₂O in the products?
View solution
Q. Ethene is burnt with 150% of excess air, what is the percentage of O₂ in the products?
View solution
Q. Ethene is burnt with 50% of excess air, what is the percentage of CO₂ in the products?
View solution
Q. 144 grams of C₅H₁₂ is burnt with 64 grams O₂, and 44 grams of CO₂ is formed, what is the percentage of excess O₂?
View solution
Q. The extent of a reaction is 10, and the stoichiometric coefficient of O₂ is 2.5, if the moles of O₂ entering the process is 45, what is the percentage of excess air?
View solution
Q. 10 moles of ethane is supplied with 49 moles of oxygen, what is the percentage of excess oxygen?
View solution
Recommended Subjects
Are you eager to expand your knowledge beyond Basic Chemical Engineering? We've handpicked a range of related categories that you might find intriguing.
Click on the categories below to discover a wealth of MCQs and enrich your understanding of various subjects. Happy exploring!