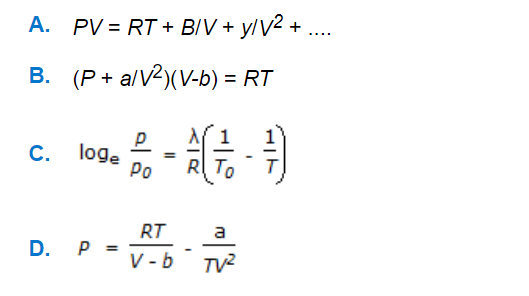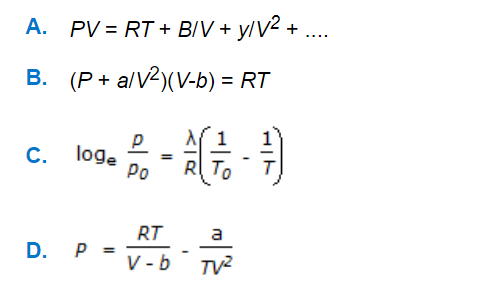Stoichiometry MCQs
Welcome to our comprehensive collection of Multiple Choice Questions (MCQs) on Stoichiometry, a fundamental topic in the field of Basic Chemical Engineering. Whether you're preparing for competitive exams, honing your problem-solving skills, or simply looking to enhance your abilities in this field, our Stoichiometry MCQs are designed to help you grasp the core concepts and excel in solving problems.
In this section, you'll find a wide range of Stoichiometry mcq questions that explore various aspects of Stoichiometry problems. Each MCQ is crafted to challenge your understanding of Stoichiometry principles, enabling you to refine your problem-solving techniques. Whether you're a student aiming to ace Basic Chemical Engineering tests, a job seeker preparing for interviews, or someone simply interested in sharpening their skills, our Stoichiometry MCQs are your pathway to success in mastering this essential Basic Chemical Engineering topic.
Note: Each of the following question comes with multiple answer choices. Select the most appropriate option and test your understanding of Stoichiometry. You can click on an option to test your knowledge before viewing the solution for a MCQ. Happy learning!
So, are you ready to put your Stoichiometry knowledge to the test? Let's get started with our carefully curated MCQs!
Stoichiometry MCQs | Page 4 of 42
Discover more Topics under Basic Chemical Engineering
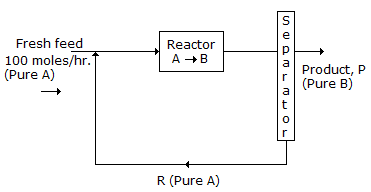
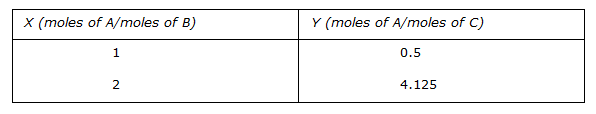
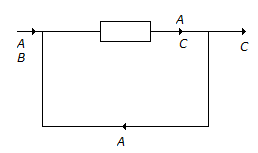
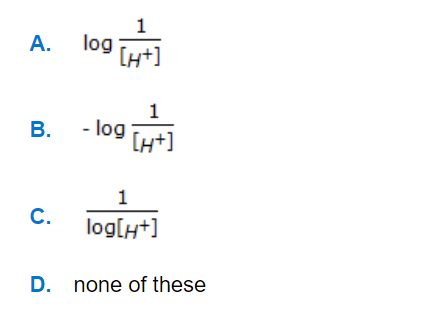
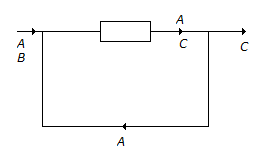
N₂ + 2O₂ ↔ 2NO₂; ΔG° = 100 kJ/mole
NO + 1/2O₂ ↔ 2NO₂; ΔG° = -35 kJ/mole
The standard free energy of formation of NO in kJ/mole is